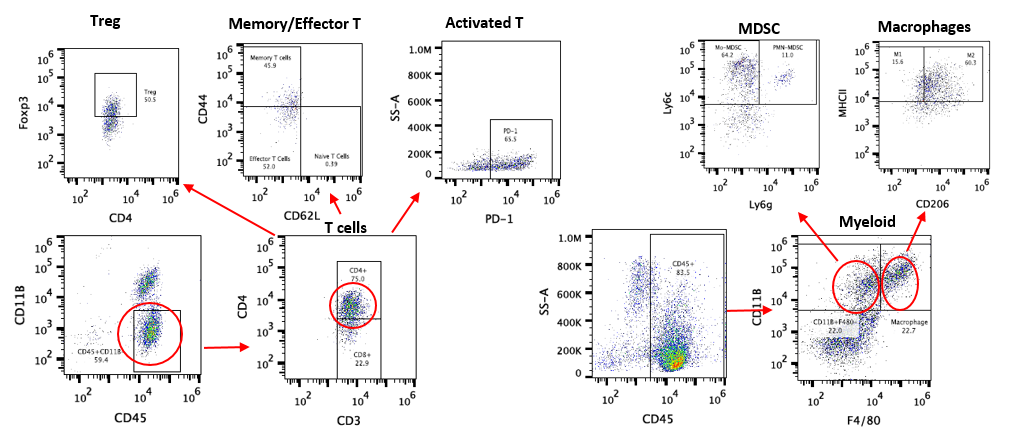
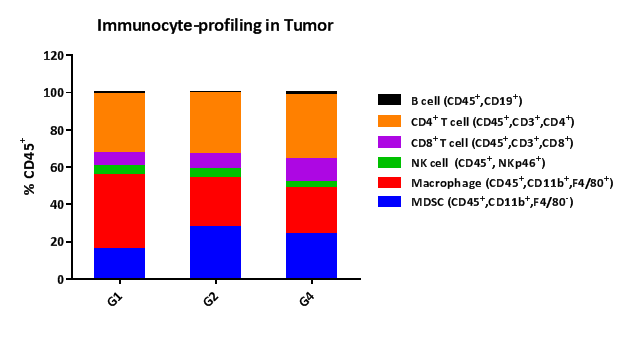PharmaLegacy provides a panel of extensively-validated syngeneic mouse models for preclinical immunotherapy research, used to interrogate novel immune-oncology treatments. We have model types including subcutaneous, orthotopic and metastatic. Our suite of syngeneic mouse models includes 22 models covering 14 cancer types, which have been validated with anti-PD-1, PD-L1 and anti-CTLA-4 antibodies. Additional models are in development.
Animal: Female BALB/c mice (from SLAC), 7~8 weeks.
Tumor Cell: CT26.WT, subcutaneous implantation.
Reagent: Anti-m-CTLA-4, Anti-m-PD-1
Dosing started on Day10 post inoculation.
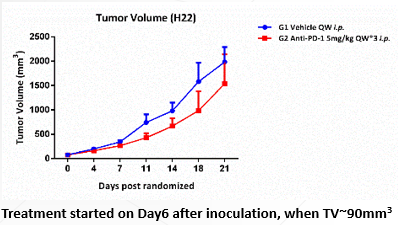
Animal: Female BALB/c mice (from SLAC), 4~5 weeks.
Tumor Cell: CT26.WT, subcutaneous implantation.
Reagent: Anti-m-PD-1 5mg/kg QW*3 i.p.
Anti-m-PD-1 1mg/kg QW*3 i.p.
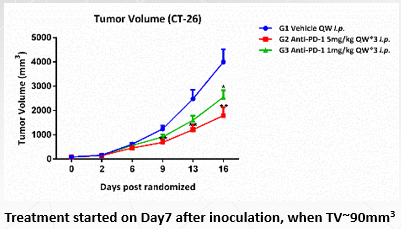
Animal: Female C57BL/6 mice (from SLAC), 5~6 weeks.
Tumor Cell: H22, subcutaneous implantation.
Reagent:Anti-m-PD-1 5mg/kg QW*3 i.p.
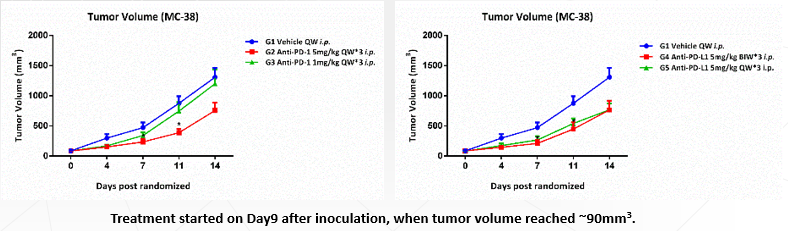
Animal: Female C57BL/6 mice (from SLAC), 5~6 weeks.
Tumor Cell: MC-38, subcutaneous implantation.
Reagent1: Anti-m-PD-1 5mg/kg QW*3 i.p.
Anti-m-PD-1 1mg/kg QW*3 i.p.
Reagent2: Anti-m-PD-L1 5mg/kg BIW*3 i.p.
Anti-m-PD-L1 5mg/kg QW*3 i.p.