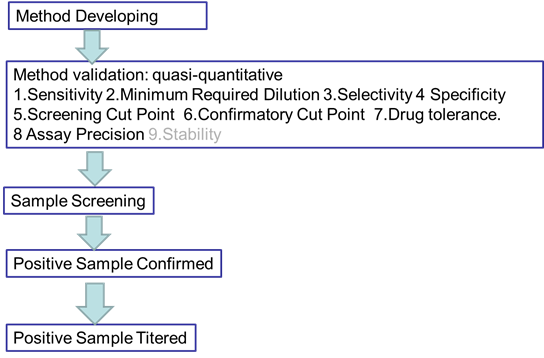
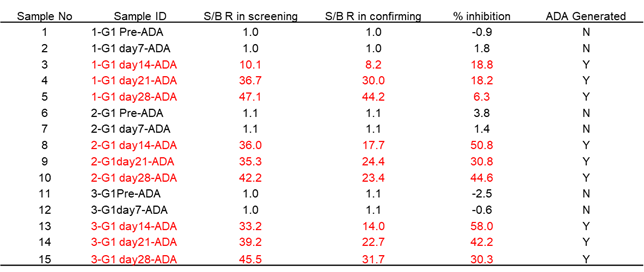
Immunogenicity is defined as the propensity of the therapeutic protein product to generate immune responses to itself and to related proteins or to induce immunologically related adverse clinical events. All biotherapeutics have the potential to elicit a host immune response that can impact their PK, efficacy and safety profiles. The immunogenicity for these novel biotherapeutics is unknown as there is limited in vivo data available. It is important, as well as a regulatory expectation, to assess immunogenicity during drug development. We can serve for the development and validation of immune assays for assessment of the immunogenicity of therapeutic protein products for pre-clinical study. It will include screening assays, confirmatory assays, titering assays, and neutralization assays. Rheumatoid arthritis (RA) is a systemic inflammatory disorder characterized by chronic inflammation of the synovium, which over time results in damage to the joints, leading to pain and disability. It occurs in approximately 1% of adults, and approximately 2.5 times more women than men are affected. Immune factors involved in the progression of RA are mainly manufactured by CD4+ T cells, monocytes, macrophages, or fibroblasts.