Inflammatory bowel disease (IBD) is a chronic relapsing and remitting inflammatory condition of the gastrointestinal tract that usually manifests as 1 of 2 distinct, but sometimes overlapping, clinical entities; ulcerative colitis (UC) and Crohn’s disease (CD). While CD is a multifocal, transmural inflammatory process that can affect any part of the digestive tract, UC is characterized by a superficial, continuous inflammation, which is limited to the large intestine. IBD is most commonly diagnosed between the third and fourth decade of life, with no difference noted between males and females.
Until recently, the clinical treatments for UC and CD have been relatively limited, essentially comprised of 5-aminosalicylic acid (ASA) compounds, steroids and azathioprine/ 6-mercaptopurine. In the 1990s, immunoregulatory agents used in IBD were expanded to include methotrexate and cyclosporine in select patient populations. The approval of infliximab (Remicade; Centocor), a chimeric monoclonal antibody directed against tumor-necrosis factor-α (TNFα), for the treatment of CD in 1998 by the FDA launched the era of biologic therapy for IBD.
IBD models can be either induced or spontaneously occurring in animals. Models include: (i) animals treated with agents that promote intestinal inflammation, (ii) rodents that have been genetically manipulated and (iii) immunodeficient animals. As the onset of inflammation is immediate and the procedure is relatively straightforward, chemically induced models of intestinal inflammation have become the most commonly used IBD animal models. At PharmaLegacy we have validated a complete set of IBD models in rodents.
· Antigen-specific T-cell responses
· Detection of tissue cytokine levels (protein, mRNA) by ELISA or real time RT-PCR
· Histopathology and immunohistochemistry in colonic tissues
· Flow cytometry analysis of cell functions including intracellular staining of cytokines
· Detection of signal transduction by western blot
With a complete set of IBD models, our scientists specialized both in immunology and pharmacology have been designing and executing various IBD studies for our clients:
1. Efficacy studies of drug compounds (clinical symptoms and disease score)
2. Proof of concept studies for early drug development:
· effects of anti-inflammation: T cell and macrophage migration
· effects of anti-autoimmune disorder
· effects of cytokine/chemokine response
· evaluation of therapeutic profile and potentials
3. Demonstration of differential regulation of disease progression
4. Investigation of the mechanisms of immune tolerance induction
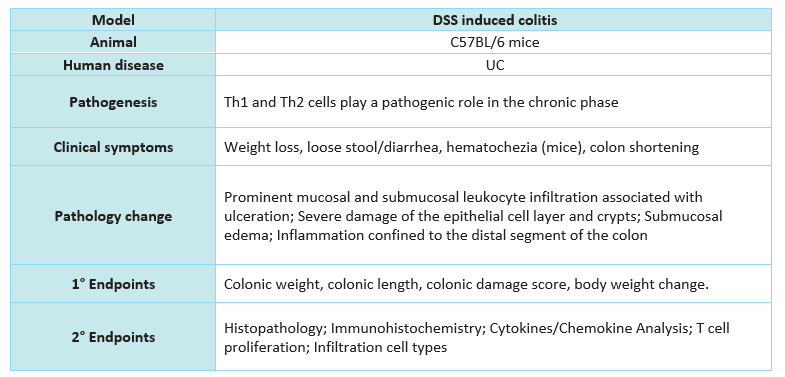
Animal: C57BL/6 mice
Reagent: Dextran sulfate sodium (DSS), Cyclosporine (CsA)
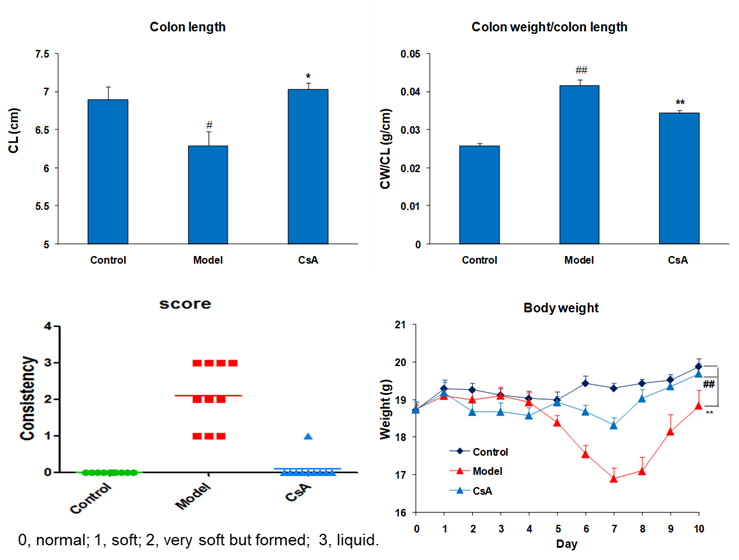
Effect of CsA on Colon Length, Colon Weight and Body Weight
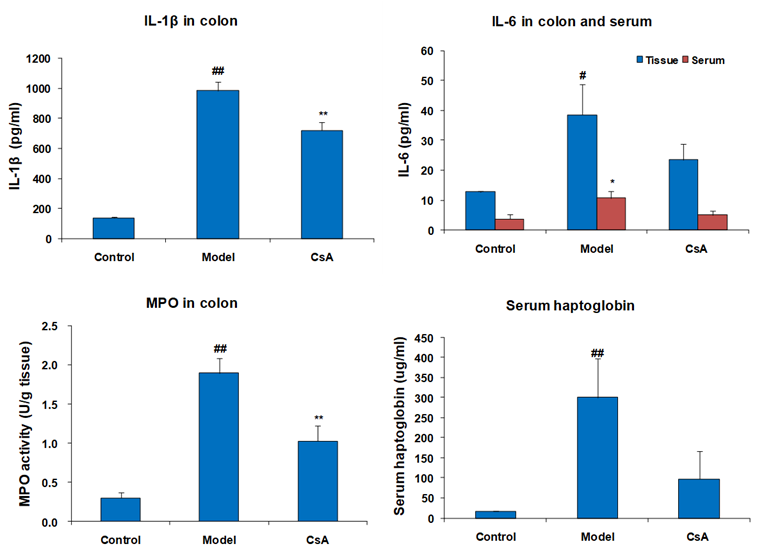
Effect of CsA on Inflammatory Markers in Colon and Serum
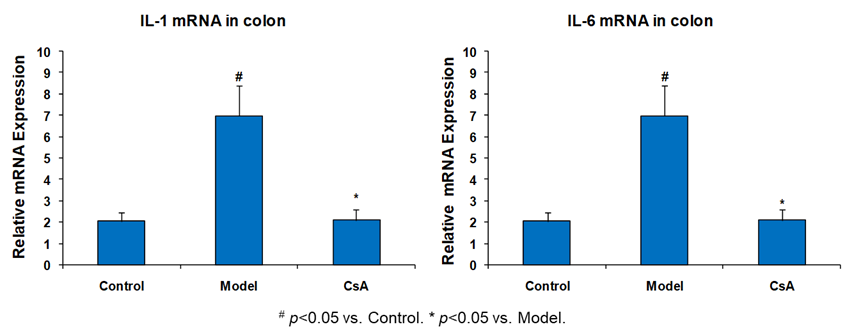
Effect of CsA on Cytokines mRNA in Colon Tissue Colon
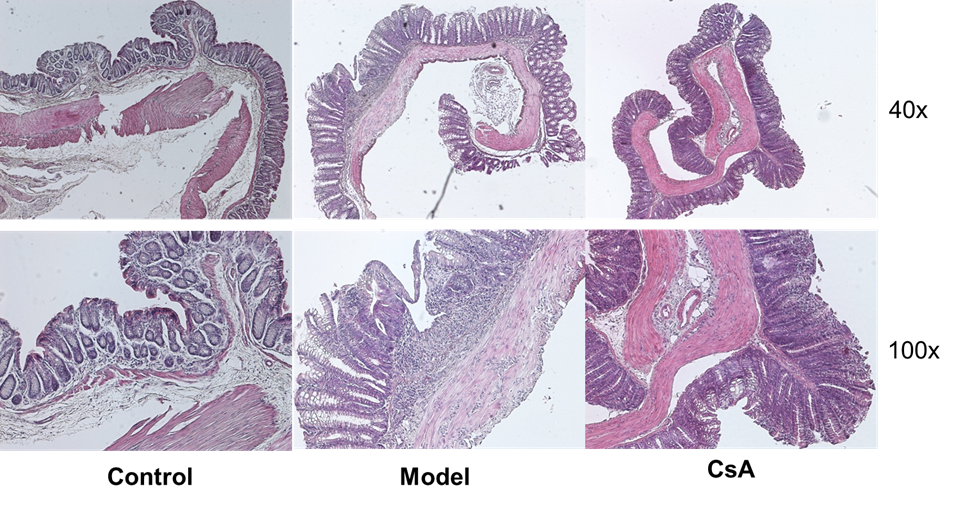
Pathology (H & E Staining)