


Liver fibrosis results from chronic injury such as viral hepatitis (especially hepatitis B and C), alcohol abuse, drugs, metabolic diseases due to overload of iron or copper, congenital abnormalities or autoimmune attack of hepatocytes or bile duct epithelium. It is characterized by the encapsulation or replacement of injured tissue by a collagenous scar. Liver fibrosis is reversible, whereas cirrhosis, the later stage of fibrosis progression, featuring regenerative nodules surrounded by fibrous bands, is generally irreversible. Liver cirrhosis leads to an impaired liver function with an increased intrahepatic resistance (portal hypertension) and the development of hepatocellular carcinoma (HCC).
Carbon tetrachloride (CCl4)-induced hepatic fibrosis and cirrhosis in rodents is a well-established and widely accepted experimental model for the study of liver fibrosis and cirrhosis. In many aspects it mirrors the pattern of human disease associated with toxic damage. For example, the α-SMA expression, Stellate cell activation and key matrix components including collagen-1, MMPs and their inhibitors TIMPs have been indicated in the pathogenesis of this model. CCl4 induction elicits a reproducible and predictable fibrotic response in liver, making it a valuable basis for preclinical pharmacology studies of anti-fibrotic and anti-cirrhotic therapeutics and the pathophysiology study of liver fibrosis-cirrhosis-HCC changes.
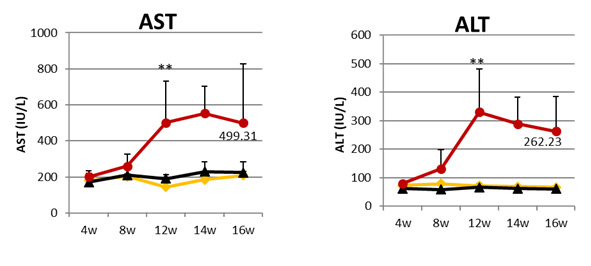
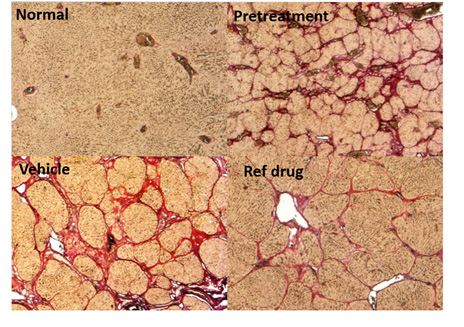
The AST and ALT in blood are increased with the duration of administration; a number of biomarker expressions are significantly increased in the liver at the end of 8 weeks; the hyperplasic nodules, surrounded by the fibrous bands, that are positively stained by Sirius Red are clearly formed in the liver at 16 weeks, but are ameliorated by Compound A treatment. (n=6, *P<0.05, **P<0.01 vs Vehicle by Bonferroni multi-comparison test)
· Serum biochemical endpoints
· Fibrotic grade evaluation in liver
· Quantification of fibrotic tissue in liver (Sirius red)
· Quantification of SMA-α positive cells
· Incidence and volume of ascites
· Vitality of activated primary stellate cells
· Morphological alterations of the hepatocytes
· Hepatic hydroxyproline, hepatic/serum glutathione, malondialdehyde (MDA) content
· Expression of cyclins, PCNA, Ki67 and BrdU for regeneration of hepatocytes
· Angiogenesis inhibitors
· Angiotensin receptor antagonists
· Vasoactive modulators
· Hepatic vasculature analysis by immunostaining of anti-vWF, VEGF-A, angiopoietin-1, angiopoietin-2, placental growth factor
· VCAM-1, ICAM-1 expression; CD11b, CD3, CD31 expression
· Apoptosis of activated hepatic stellate cells
Bio-marker analysis of involved signaling pathways