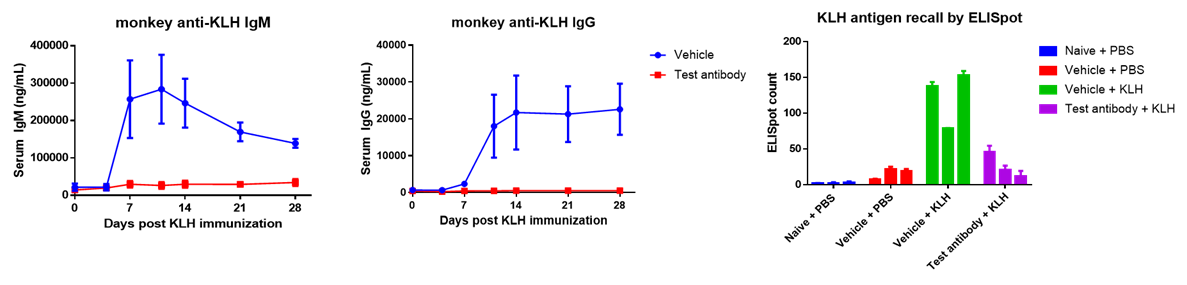
TDAR to an antigen is a comprehensive immune function assay evaluating various aspects of immune responses, including antigen processing and presentation, B and T lymphocyte interactions, antibody production and cytokine-dependent isotype class switch (i.e., IgM to IgG specific antibody response). The use of a TDAR assay is therefore endorsed by regulatory agencies as a "default" immune function test for evaluation of the immuno-toxicity potential of new drug candidates.
TDAR is also employed to evaluate investigational drug efficacy in preclinical pharmacology studies, provide evidence of biological impact in clinical trials, and evaluate primary or secondary immunodeficiency diseases. TDAR model in NHP provides a chance to overcome species specificity issue that biologics drug may have. NHP is also used in TDAR if it is deemed to be a more appropriate species for a particular small molecule (e.g., similarity of metabolism, better exposure, or immunity-related finding in standard toxicity studies only present in NHP).